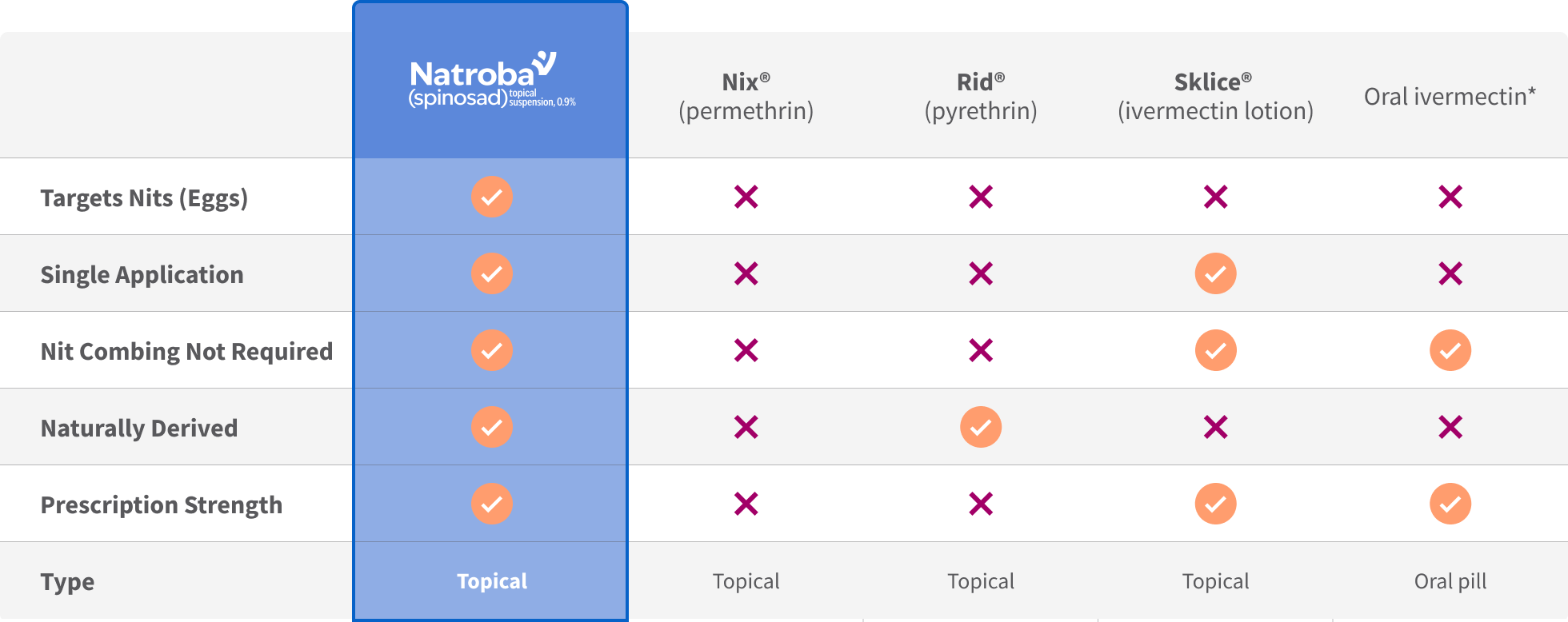
Kills head lice that infest the scalp and their nits (eggs), which cause itching and can lead to reinfestation.1
Just one easy application—no repeat treatments needed for most patients.1
In studies, Natroba™ (Spinosad) was superior to other products like Nix®, with no nit combing required.1,3
-
Targets Head Lice at the Source
Works where head lice live—on the scalp and in the hair.1
-
One 10-Minute Application
Specifically designed to kill both lice and their eggs (nits).1
-
No Nit Combing
Most patients need only one treatment, skipping hours of nit combing.1
-
Relieves Discomfort
As lice and eggs are eliminated, itching and irritation fade—so you can get back to normal, fast.

Attentive parents choose Natroba™ (Spinosad) head lice medication because it offers important advantages over many other head lice treatments, both pharmacy-aisle and prescription.
In studies, up to
of patients were head lice-free.3
“Super lice” are resistant to common pharmacy-aisle treatments like permethrin and pyrethrin.4
Over 98% of head lice in the U.S. now have gene mutations making them hard to treat with traditional products.4
Natroba™ (Spinosad) is clinically proven to be effective—even for families who have tried and failed with other products.3
-
Kills Both Head Lice and Nits
The only head lice treatment clinically proven to eliminate both adult head lice and their nits (eggs), helping prevent reinfestation and making it a one-and-done solution.1,2
-
Single Application
Most people are head lice-free and back to life after just one application. No repeat dosing or stressful routines as required by other head lice treatments.1,2
-
Proven Even When Others Fail
Natroba™ (Spinosad) is clinically proven to be superior to Nix® (permethrin).3
-
Naturally Derived
The active ingredient, spinosad, is derived from a soil bacterium. Natroba™ (Spinosad) is safe and FDA-approved for children as young as 6 months.1,3 It works only on the scalp and is not absorbed throughout the body, like some other head lice treatments. Natroba™ (Spinosad) has been trusted by doctors and families for over 10 years.
*Oral ivermectin is not FDA-approved for head lice but may be prescribed off-label.
Click 'Get Natroba Now' to have Natroba™ (Spinosad) delivered right to your home
No repeated, ineffective, messy home remedies
No need for follow-up visits to head lice clinics
Many parents first try home remedies hoping for a quick fix. But common DIY approaches—such as mayonnaise, olive oil, or essential oils—have not been proven to work and only waste valuable time. Head lice is a medical condition, requiring a prescription-strength medication.
Head lice clinics are expensive, have long waits, and typically require return visits to address eggs not caught by nit combing. Not a good solution for parents.

“My children kept getting lice for years. With their long curly thick hair it was impossible to get them all out. Called our family doctor, he prescribed Natroba over the phone. Was due for a 7-day refill and haven't had to use it!!! Amazing stuff, call your doctor if you’re having issues. Don't pay for expensive treatments, it won't work! Natroba really does.”

“I have three daughters, yes 3! All have long hair and we contracted lice. I kept treating, combing, vacuuming, washing, over and over, and then do a random check in between and every time they had new nits. I was beyond stressed and at my wit's end. I finally asked the doctor if there was something he could call in. We did the Natroba and it was a life saver! It worked after one treatment! I went ahead and did a follow up because there was a refill on the script but never had any evidence of any more lice after the first dose. Best stuff ever!”
Natroba™ (Spinosad) is widely covered by insurance plans and Medicaid. Typically, your out-of-pocket cost will be lower than most pharmacy-aisle treatments and far lower cost than nit clinic visits.
Natroba™ (Spinosad) is the brand name and Spinosad is the authorized generic version. Which version you get will depend on your doctor’s preference and your insurance. The formulations are identical and equally safe and effective.
Get back to life,
fast and easy.
Order Natroba™ (Spinosad) head lice medicine online for fast, discreet, prescription delivery right to your door.
-
How fast does Natroba™ (Spinosad) work?
Natroba™ (Spinosad) is designed to work with a single 10-minute treatment. Most patients are head lice-free after one application. In the rare case that live head lice are seen after treatment, Natroba™ (Spinosad) can safely be used again 7 days after the first treatment.1
Learn how Natroba™ (Spinosad) works -
Is a prescription required for Natroba™ (Spinosad)?
Yes, ask your doctor for Natroba™ (Spinosad) or you can get a prescription online here – fast and easy.
Start your online visit -
How much does Natroba™ (Spinosad) cost?
Many families only pay their usual insurance co-pay, or an online cash purchase option is also available.
-
Is Natroba™ (Spinosad) covered by insurance?
Yes, Natroba™ (Spinosad) is covered by many major insurance plans.5
-
Is Natroba™ (Spinosad) safe for children?
Yes, Natroba™ (Spinosad) is FDA-approved for children 6 months and older for treating head lice. It only targets the bugs, not the body. That means the active ingredient Spinosad is not getting absorbed into your skin.1,3
See safety info -
What if I tried other head lice treatments first?
Over-the-counter pharmacy-aisle medicines are typically ineffective. Natroba™ (Spinosad) can be safely used anytime and works even after other head lice medications fail.6
See how Natroba™ is different for head lice -
Does Natroba™ (Spinosad) kill the head lice eggs (nits)?
Yes.3
-
Does Natroba™ (Spinosad) require nit-combing?
No. Most other head lice medications require nit-combing. Natroba™ (Spinosad) does not, making it the quick and easy solution.3
-
How do you get head lice?
Head lice are spread primarily by direct head-to-head contact with an infected individual. They crawl but cannot fly or jump. Head lice can also occasionally spread through sharing items like hats, combs, or brushes, but this is less common.7
-
How do I know if my child (or I) have head lice?
Symptoms include itching on the scalp, a crawling sensation, and sometimes visible head lice or eggs (nits) on the hair. A fine-toothed nit comb can help find live head lice or nits close to the scalp, especially behind the ears and at the nape of the neck.7
-
How do you treat head lice?
Natroba™ (Spinosad) typically works with a single 10-minute treatment. Most patients are head lice-free after one application and no nit-combing.1,8
-
What are head lice?
Head lice are tiny parasitic insects (about the size of a sesame seed) that live on the human scalp and feed on blood. They are common, especially among school-aged children, but do not transmit disease.7
-
Are head lice contagious?
Yes, head lice spread primarily through close, direct head-to-head contact, which makes them especially common among children who play closely together. Head lice do not fly or jump.7
-
What do head lice look like (including nits/eggs)?
Adult head lice are tiny, tan-colored insects. Their eggs (nits) resemble small dots firmly attached to the hair shaft near the scalp. Empty eggshells (from hatched nits) may appear further from the scalp.1,8
-
How do you prevent head lice?
Prevent head lice by avoiding head-to-head contact, especially during play and group activities, and by not sharing personal items like hats, brushes, helmets, or towels with someone who has head lice.7
-
Can head lice live on pets or in the environment?
No, head lice live only on humans and feed on human blood. Pets do not get or spread head lice. Head lice that fall off a person usually die within 1-2 days if they can’t feed.7

